may 19, 2020 - Philips
Philips launches HealthSuite Clinical Trial Accelerator to help life science organizations run more flexible, patient-centric clinical trials
Royal #philips, a global leader in #health technology, today announced Clinical Trial Accelerator on #philips HealthSuite. This new tailored set of capabilities allows life science organizations to integrate, analyze and store clinical and patient-reported data from multiple sources, providing actionable insights for better, faster decision-making.
Philips HealthSuite is an integrated, modular set of standards-based capabilities that support the development of digital propositions across the #health continuum, encompassing HIPAA compliant, secure cloud capabilities. Clinical Trial Accelerator enables patient-centric trials at home, managing the collection of data while providing the security and compliance expected from both patients and life science organizations.
Life science organizations face continued pressure to run more efficient, cost-effective clinical trials. Empowering patients and making it easier for them to stay connected and contribute to a trial can have a significant impact on compliance rates and overall success. At the same time, collecting data and identifying actionable insights while ensuring the security and compliance of patient information is key. Clinical Trial Accelerator is designed to address these challenges, helping life science organizations reach their goals faster and supporting greater flexibility in trial design.
"Our innovative, flexible cloud platform leverages our global healthcare capabilities and is supported by our compliance, security and interoperability expertise". Dale Wiggins, General Manager for the HealthSuite digital platform at #philips.
“Philips HealthSuite already provides cloud-based services and tools to a wide array of healthcare organizations, and with Clinical Trial Accelerator we have created a tailored set of capabilities to support life science organizations,” said Dale Wiggins, General Manager for the HealthSuite digital platform at #philips. “Our innovative, flexible cloud platform leverages our global healthcare capabilities and is supported by our compliance, security and interoperability expertise.”
Tailored capabilities to accelerate clinical trials
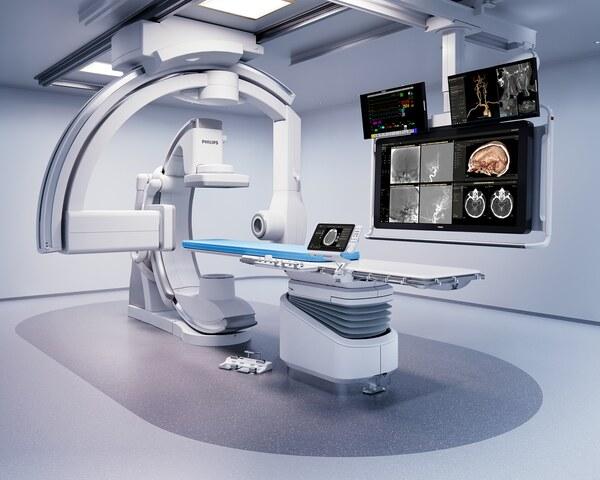
Other HealthSuite capabilities include an AI Analytics Workbench for enhanced decision making and standards-based interoperability for Digital Imaging and Communication in Medicine (DICOM) data, as well as the HealthSuite Clinical Data Lake, a new scalable micro-service that acts as a centralized big data repository for high-volume clinical data collection studies and includes controls to curate and manage data in a manner that addresses regulatory requirements.
These HealthSuite capabilities offer companies cloud-based services and technical tools that meet privacy, security and regulatory requirements that they can use to develop and run a new generation of connected healthcare applications. Unlike other digital platforms, HealthSuite is purpose-built to solve the complex challenges of healthcare, featuring capabilities to manage device data, collect personal and #health data, store and share data securely, analyze data, build and deploy AI models, and create solutions on the cloud.
In addition, these HealthSuite capabilities power a wide range of both #philips and 3rd party connected healthcare applications in areas including image-guided therapy, sleep and respiratory care, ophthalmology, telehealth and self-medication. For 3rd party collaborations, #philips is the provider of the relevant cloud-based healthcare IT infrastructure and is not involved in the commercialization of the products and services of these companies. More information on #philips HealthSuite can be found at www.philips.com/healthsuite.











 Share
Share Share via mail
Share via mail  Automotive
Automotive Sport
Sport Events
Events Art&Culture
Art&Culture Design
Design Fashion&Beauty
Fashion&Beauty Food&Hospitality
Food&Hospitality Technology
Technology Nautica
Nautica Racing
Racing Excellence
Excellence Corporate
Corporate OffBeat
OffBeat Green
Green Gift
Gift Pop
Pop Heritage
Heritage Entertainment
Entertainment Health & Wellness
Health & Wellness